By Sean Murphy and Matthew Perrone
A federal judge in Texas will hear arguments Wednesday in a high-stakes court case that could threaten access to abortion medication and blunt the authority of U.S. drug regulators.
Judge Matthew Kacsmaryk is weighing a lawsuit from Christian conservatives aimed at overturning the Food and Drug Administration’s more than 2-decade-old approval of the abortion pill mifepristone. The drug, when used with a second pill, has become the most common method of abortion in the U.S.
There is essentially no precedent for a lone judge overruling the scientific decisions of the FDA. And legal experts have warned of far-reaching consequences if judges begin second-guessing FDA decisions on drug safety and effectiveness.
Wednesday's hearing is the first in the case, which is being intensely tracked by groups on both sides of the abortion issue after last year's reversal of Roe v. Wade. However, there was little advance notice of the high-profile session, which only appeared on the public online docket late Monday after news reports raised concerns about a lack of transparency in the proceedings.
Kacsmaryk told attorneys in the case Friday that he would delay the filing to minimize threats and possible protests, a development first reported by The Washington Post. He also asked the lawyers not to disclose the date of the hearing, according to a transcript of the meeting released Tuesday.
Such actions by a judge are highly unusual because court proceedings are almost always open to the public and transparency is an underlying assumption of the American judicial system.
Kacsmaryk, appointed by President Donald Trump, formerly worked as an attorney for a Christian legal group and has written critically of laws allowing abortion. Supporters of abortion rights say conservatives are steering cases to his courtroom because they believe he will rule in their favor.
On Wednesday, Kacsmaryk will hear arguments in Amarillo from the Alliance for Defending Freedom — which filed its lawsuit on behalf of several anti-abortion groups and physicians — as well as federal attorneys representing the FDA. The drug's manufacturer, Danco Laboratories, is also a party in the case and is set to argue for keeping its pill available.
The alliance is seeking an injunction that would force the FDA to revoke its approval of mifepristone. But it's unclear how quickly that could happen or what the process would entail. The FDA has its own procedures for revoking drug approvals that involve public hearings and scientific deliberations, which can take months or years.
If Kacsmaryk rules against the FDA, federal attorneys are expected to swiftly appeal the decision and seek an emergency stay to stop it from taking effect while the case proceeds.
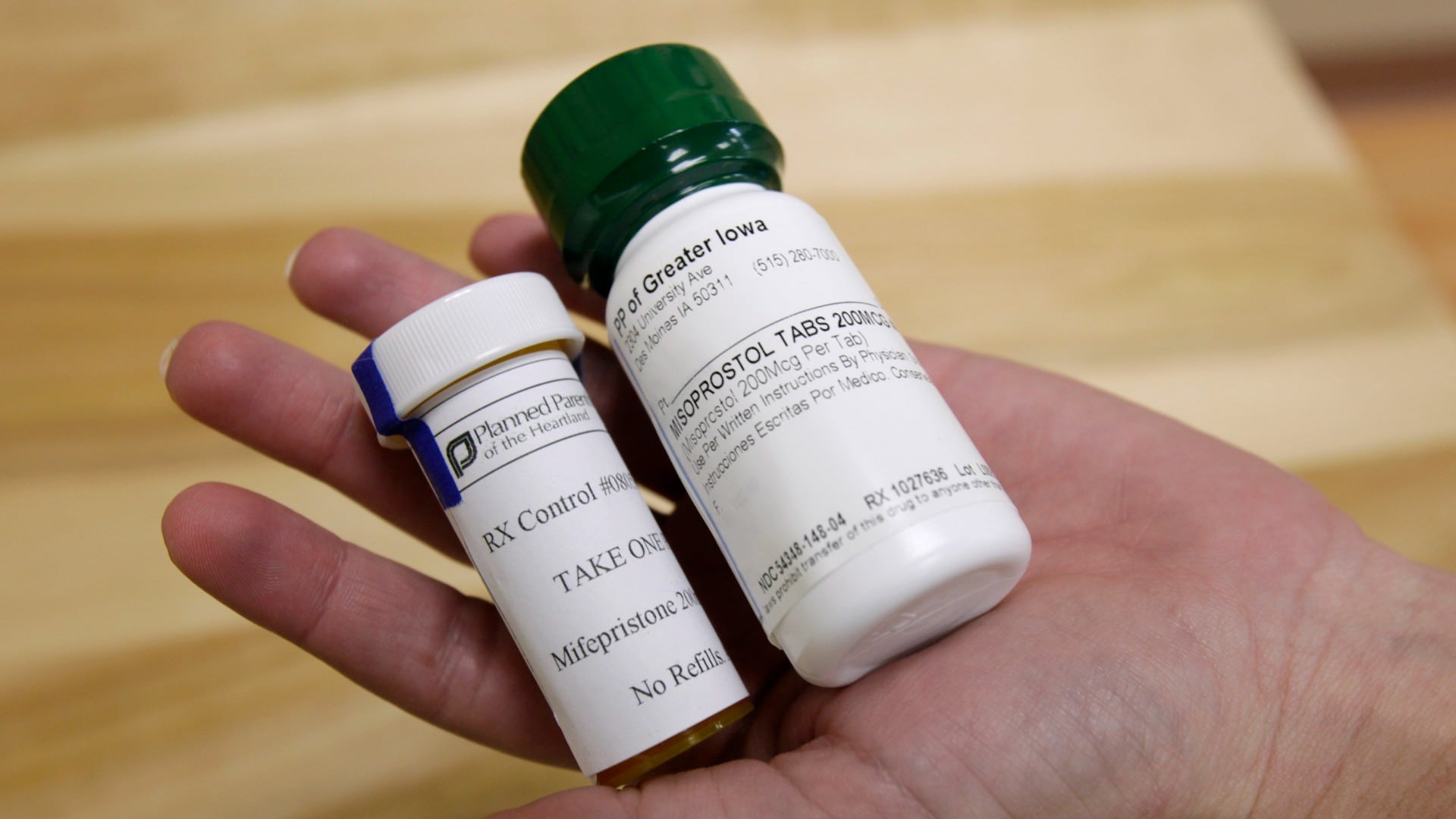
Mifepristone is part of a two-drug regimen that has been the standard for medication abortion in the U.S since 2000. If mifepristone is sidelined, clinics and doctors that prescribe the combination say they plan to switch to using only the second drug, misoprostol. That single-drug approach is slightly less effective at ending pregnancies, though it is widely used in countries where mifepristone is illegal or unavailable.
The Texas lawsuit alleges that the FDA's approval of mifepristone in 2000 was flawed for several reasons, including an inadequate review of the pill's safety risks. The suit also challenges several later FDA decisions that loosened restrictions on the pill, including eliminating a requirement that women pick it up in person.
Lawyers for the FDA have pointed out that serious side effects with mifepristone are rare and the agency has repeatedly affirmed the drug's safety by reviewing subsequent studies and data. Pulling the drug more than 20 years after approval would be “extraordinary and unprecedented,” the government stated in its legal response.
Typically, the FDA’s authority to regulate prescription drugs has gone unchallenged. But more than a dozen states now have laws restricting abortion broadly — and the pills specifically — following last year’s Supreme Court decision overturning Roe v. Wade.
Lawsuits challenging state restrictions, including those in North Carolina and West Virginia, are progressing separately and are expected to continue for years.
___
Perrone reported from Washington.
___
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.
UPDATES: Minor edits. Hearing scheduled for 10 a.m. EDT.